
Medical & Pharmaceutical Grade O-ring Materials
In the fields of medicine and pharmaceuticals, the quality and safety of materials used are of paramount importance. Medical and pharmaceutical grade materials are designed and manufactured to meet rigorous standards, ensuring that they do not compromise patient health. In this web page, we will delve into the significance of medical and pharmaceutical grade materials, common application considerations, and discuss the USP Class VI 87 & 88 standards that govern these materials.
With Canyon Components, you can be sure that high quality materials that meet you needs are always readily available. Custom & standard Medical & Pharmaceutical Grade Materials available now!
Check with one of Canyon’s helpful product engineers for an expert material and manufacturing recommendation.

Medical & Pharmaceutical Materials Available
Thousands of Medical & Pharmaceutical Grade Materials are available now with Canyon Components! Some common Medical & Pharmaceutical Grade Materials include the following.
This table shows many of our standard materials and links out to our O-ring store. Get in touch with us if you need a custom gasket, custom molded part, or non-standard geometry!
Filter by
Temperature Search (°C)
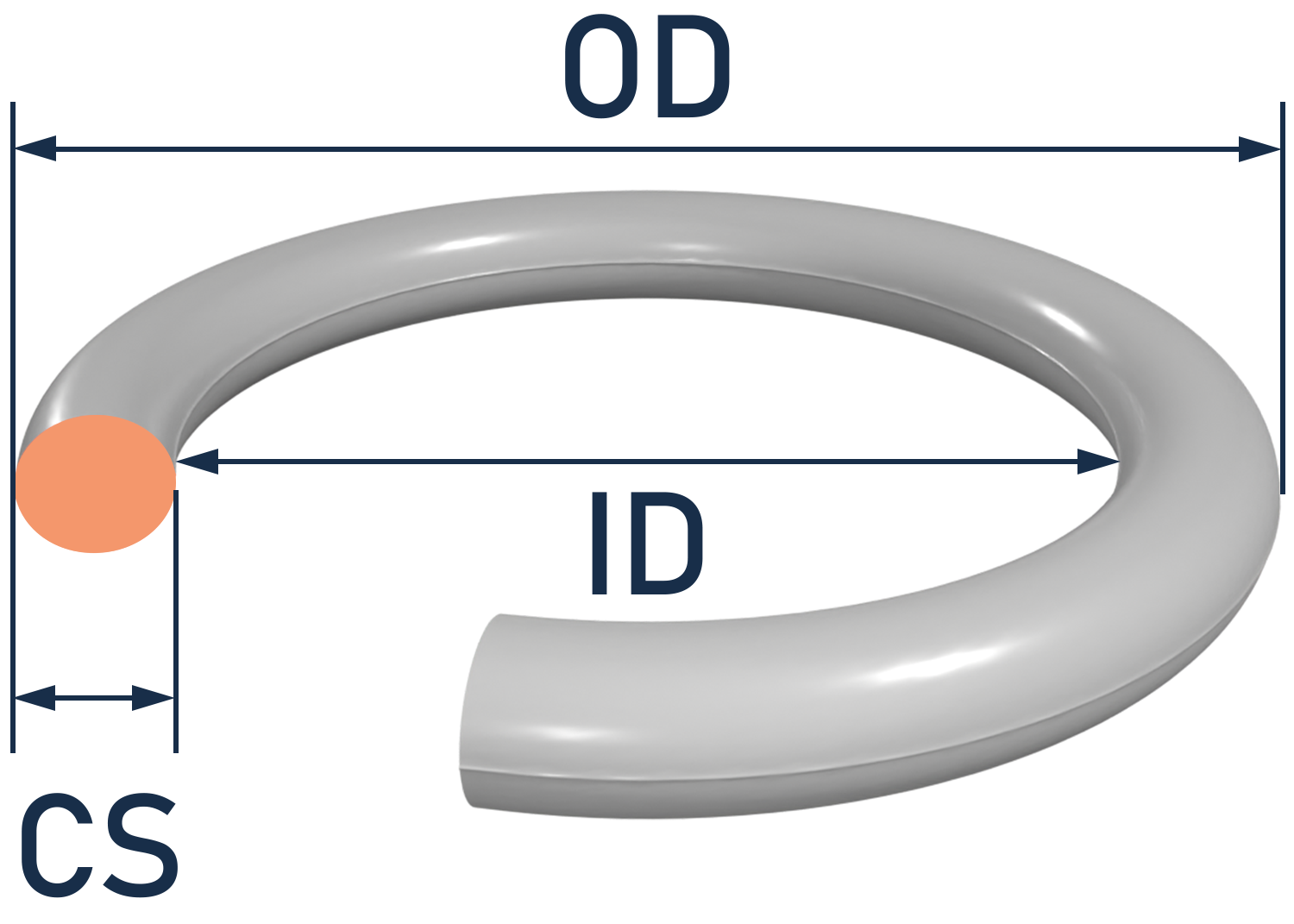
Please consult a Canyon Components Engineer about your specific application and we will use our decades of experience to formulate a solution that fits your need.
What are Medical & Pharmaceutical Grade Materials?
- Medical and pharmaceutical grade materials are substances, components, or equipment specifically engineered to meet the stringent requirements of the healthcare and pharmaceutical industries. These materials must be biocompatible, non-toxic, and free from contaminants to ensure patient safety and the efficacy of pharmaceutical products.
Common Considerations
- Biocompatibility: Materials must be biocompatible, meaning they do not cause adverse reactions when in contact with living tissues, blood, or bodily fluids.
- Purity and Sterility: Materials should be pure and sterile to prevent contamination of pharmaceutical products and minimize the risk of infections or adverse reactions in medical applications.
- Chemical Compatibility: Materials must be chemically compatible with the drugs, chemicals, and processes they come into contact with, ensuring product stability and efficacy.
- Traceability: Materials should be traceable to ensure quality control, batch tracking, and recall procedures in case of issues.
- Regulatory Compliance: Compliance with regulatory standards, such as the USP Class VI 87 & 88, is essential to meet the strict requirements of the healthcare and pharmaceutical industries.
Key Standards
- USP Class VI: USP Class VI materials undergo an array of cytotoxicity tests that aim to certify that a material is biocompatible by proving that it doesn't leach any chemicals that have harmful reactions or long term bodily effects. These tests are designed to assess systemic biological reactivity when the material in question makes contact with mammalian cells. It's commonly used for O-rings, gaskets, & custom molded parts.
- USP Class VI <87>: USP Class VI <87> is a set of in-vitro cytotoxicity tests involving agar diffusion, elution assays, and direct contact. It's commonly used for O-rings, gaskets, & custom molded parts.
- USP Class VI <88>: USP Class VI <88> is a set of in-vivo cytotoxicity tests involving direct implantation, intracutaneous injection, and systemic injection studies. It's commonly used for O-rings, gaskets, & custom molded parts.
Please consult a Canyon Components Engineer about your specific application and we will use our decades of experience to formulate a solution that fits your need.
Medical & Pharmaceutical
USP Class VI Materials
Explore USP Class VI materials for medical devices, pharmaceuticals, and food processing, ensuring safety and purity.
ISO 10993-1
Discover ISO 10993-1 materials, certified for biocompatibility in medical devices, ensuring safety and performance.
Vacuum Baking & Cleaning
Explore our specialized services: vacuum baking for material degassing, ultrasonic cleaning for precision parts, and custom bagging for product protection. Request a quote today!
Clean Room Manufactured Materials
Discover clean room manufactured material features, applications, and how they meet strict cleanliness standards in medical, pharmaceutical, and semiconductor environments.
Medical & Pharmaceutical Materials Available
Click here to see some of our most commonly served industries!
Each of these materials has its own advantages, limitations, and cost implications. The choice of material usually depends on factors like chemical compatibility, temperature, pressure, dynamic or static use, and specification requirements.
Canyon Components strives to meet all customer service requests. Feel free to contact Canyon Components engineering and let our knowledgeable staff help you design the perfect part for your needs.
Please consult a Canyon Components Engineer about your specific application and we will use our decades of experience to formulate a solution that fits your need.
Back to Industries Hub
Get A Quote Now!

Groove Design References
Learn More
Coatings, Packaging, & Other Services
Learn More
Custom Parts & Custom O-rings
Learn More
FAQ
Sealing components for medical and pharmaceutical applications must meet far more than simple mechanical requirements. They are part of systems that handle sterile fluids, life-saving drugs, and complex biological materials. The slightest contamination or material degradation can compromise patient safety or disrupt production. Canyon Components provides O-rings, gaskets, and custom-molded seals that meet the demanding performance and purity standards of the healthcare and pharmaceutical industries. Our materials include Kalrez®, Canrez®, Chemraz®, and Parker® compounds in FFKM, FKM, EPDM, silicone, and USP Class VI formulations, all tested for biocompatibility, extractables, sterilization resistance, and compliance with FDA, USP, and ISO regulations.
What standards govern medical and pharmaceutical sealing materials?
Sealing materials used in these sectors must comply with multiple international standards that ensure safety, biocompatibility, and traceability. Key requirements include USP Class VI testing for biological reactivity, ISO 10993 testing for cytotoxicity and systemic toxicity, and FDA CFR 21.177.2600 compliance for materials used in contact with food or pharmaceutical products. Additional approvals such as NSF 51 and 61, 3A Sanitary, and European Pharmacopoeia 3.1.9 may apply depending on the end-use. Canyon Components provides full documentation packages, including Certificates of Conformance, batch traceability, and test reports, to verify that each material meets or exceeds applicable standards. These materials are manufactured and packaged under controlled conditions to ensure consistency and purity at every stage of production. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
How are medical-grade O-rings and gaskets manufactured differently from standard seals?
Medical-grade sealing components undergo more stringent manufacturing and quality control processes than general-purpose parts. Canyon Components produces many of these seals using clean compounding, molding, and post-curing techniques that eliminate contaminants, extractables, and residual chemicals. Typically we use dedicated tooling for medical-grade materials to prevent cross-contamination from industrial compounds. After molding, parts can be post-cured, plasma-treated, or washed in deionized water to achieve low particulate and low volatile residue levels. Components destined for sterile environments are further cleanroom packaged under ISO Class 7 or Class 8 conditions, ensuring they arrive contamination-free and ready for use in validated manufacturing or patient-contact systems. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
Which materials perform best in pharmaceutical and bioprocessing environments?
Material performance depends on both process media and sterilization method. FFKM materials like Canrez® and Kalrez® provide unmatched chemical resistance for use with solvents, acids, and sanitizing agents in aggressive clean-in-place (CIP) and steam-in-place (SIP) cycles. EPDM is preferred for water and steam handling systems because of its excellent hot-water and peroxide resistance. Silicone and fluorosilicone compounds are used in applications that require extreme temperature flexibility or transparency. FKM (Viton®) offers excellent sealing strength and resistance to oils and pharmaceutical solvents. Above all, Canyon Components offers compounds in each of these material categories that are USP class VI. Canyon Components helps customers evaluate each environment, whether it involves exposure to ethanol, saline, or sterilizing steam, to select the compound that provides the right balance of purity, elasticity, and chemical stability. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
How does Canyon Components ensure biocompatibility and cleanliness?
Every batch of medical and pharmaceutical-grade materials is manufactured under strict process controls to maintain biological safety and cleanliness. Canyon Components uses raw materials certified to be free from animal-derived substances and latex to minimize allergen risk. Our biocompatible compounds are tested to verify that they do not cause cytotoxic, irritant, or systemic responses when exposed to living tissue. After molding, parts undergo cleaning procedures that remove surface residues and particulates. Components can also be sterilized or gamma-compatible upon request. These measures ensure that every seal meets the purity standards required for direct contact with drug products, biological materials, or medical fluids. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
What sterilization methods can Canyon Components’ materials withstand?
Our materials are designed to maintain their sealing performance after repeated sterilization cycles. FFKM and FKM compounds handle the broadest range of sterilization environments, including high-pressure steam and chemical disinfectants. Silicone and EPDM exhibit excellent recovery and elasticity after steam autoclaving or ethylene oxide exposure. For gamma and e-beam sterilization, Canyon Components provides formulations with enhanced radiation stability to prevent brittleness or discoloration. We can also perform cycle testing to verify that materials retain mechanical integrity and compression set resistance after hundreds of sterilization rounds, ensuring consistent sealing performance throughout the life of the component. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
Can Canyon Components supply seals for single-use and disposable systems?
Yes. Single-use bioprocessing systems are increasingly replacing traditional stainless-steel equipment to reduce cleaning costs and contamination risk. Canyon Components supplies pre-sterilized, bagged, and labeled seals and gaskets suitable for single-use assemblies. These parts are produced from low-extractable silicone, EPDM, or TPE materials that meet USP and FDA standards. For tubing connections, manifolds, and disposable sensor housings, we can provide custom-molded geometries that maintain secure seals without adhesives or welds. Each batch is validated for dimensional accuracy, material compatibility, and sterility assurance, supporting rapid integration into single-use pharmaceutical production lines. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
How are Canyon Components’ seals used in medical devices?
Our medical-grade seals are used in diagnostic equipment, infusion systems, surgical instruments, respiratory devices, and various other medical devices. They provide consistent sealing in both dynamic and static applications where cleanliness and material stability are essential. For drug delivery systems and analytical equipment, we provide precision micro O-rings molded from USP Class VI materials. In reusable medical devices, we offer long-life elastomers and coatings that resist bodily fluids and sterilization cycles without hardening or swelling. Canyon Components also works with OEMs to co-develop custom seal geometries that optimize flow paths, minimize dead space, and maintain biocompatibility throughout repeated use. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
What documentation and traceability options are available?
Canyon Components provides full traceability from raw material to finished part. Each shipment can include Certificates of Conformance, lot-specific batch records, USP and FDA compliance certificates, and detailed material data sheets. Our documentation supports audit readiness and regulatory validation for FDA and ISO 13485 quality systems. For global customers, we also offer EU 1935/2004 and REACH-compliant documentation to streamline cross-border regulatory approvals. This level of traceability ensures that every O-ring, gasket, or molded component can be confidently integrated into validated medical or pharmaceutical processes. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!
What makes Canyon Components a trusted partner for the medical and pharmaceutical industries?
Canyon Components combines material science expertise with manufacturing precision and regulatory understanding. We supply materials that meet the most stringent international standards while maintaining flexibility in design, tooling, and production. Our cleanroom packaging, extensive testing capabilities, and rapid prototyping services enable customers to bring compliant medical and pharmaceutical products to market faster. Whether you need FFKM seals for sterilization lines, silicone O-rings for diagnostic systems, or custom-molded parts for patient-contact applications, Canyon Components ensures that every component performs flawlessly, meets global compliance requirements, and supports the integrity and safety of critical healthcare processes. If you have any questions or require design assistance, feel free to reach out to the Canyon Components engineering team!




